

Clinical Trial Expertise in Global Health: Bringing Science to Patients
With over 20 years of clinical research and operational experience, Swiss TPH has a unique expertise in clinical trials. We are dedicated to the design, implementation, management and monitoring of Phase I to Phase IV clinical trials with a focus on poverty related diseases. As both a sponsor and service provider, we understand the barriers to delivering clinical trials in difficult settings and offer strategic, customized solutions with the aim to deliver high quality data for clinical trials, putting the patient at the center of our efforts.
Our expertise lies in the development and evaluation of drugs, diagnostics and vaccines and finding novel solutions for non-communicable and neglected tropical diseases. We do this through:
⇒ Local know-how of regional context
⇒ Understanding of patient pathway
⇒ Academic and scientific innovation
⇒ Clinical expertise and management
⇒ Global network of partners and collaborators
Our Expertise
Through our multidisciplinary teams, large network of collaborators and local and global experts, Swiss TPH supports a diversity of clients. Our long-standing expertise allows us to continuously adapt and develop expertise from both investigator-initiated studies as well as regulatory trials. Our services include:
Trial Management of Multinational Studies
Our global, multidisciplinary clinical research professionals are dedicated to advancing clinical trials. Through our combined clinical, research and local expertise with long-standing partners in an extensive global network of regional collaborators, we support the management, conduct and monitoring of multi-center studies to deliver high-quality, consumer-focused clinical trial results.



Study Design and Adaptability
Good clinical trial design is key to optimizing study conduct. Performing clinical trials based on a sound understanding of clinical practice facilitates the implementation of evidence-based practice and adaptability. Through a multifaceted approach and diverse expertise from clinical staff, pharmacists, biologists, epidemiologists, data managers and statisticians, we aim to optimize clinical trials robustness and success.



Feasibility and Study Site Evaluation
It’s estimated that more than 90% of clinical trials fail due to poor patient recruitment, inefficient clinical investigator selection, poor understanding of ethical and local regulatory aspects of the selected site for conducting the study, cost incurred due to delays and more. Swiss TPH has been successfully facilitating the feasibility, set-up, project management and monitoring of epidemiological studies and clinical trials(Phase I-IV) in low-income settings and emerging economies for 20 years.



Strengthening Capacity Building
Through our combined clinical, research and local expertise and an extensive global network of regional collaborators, Swiss TPH embeds capacity building throughout all our projects as part of our commitment to sustainable development. In addition, we provide mentorship and training support for people at the European Centre for Clinical Research, the University of Basel and we are an active member of the WHO Special Programme for Research and Training in Tropical Diseases.



Planning and Conducting Phase I-IV Studies
Swiss TPH combines translational and clinical research to advance laboratory discovery and apply the findings within communities. By working across medical parasitology to infection biology, our researchers collaborate with international partners to bridge the gap between bench and bedside, ensuring that safe, effective and innovative treatments reach patients as quickly as possible.



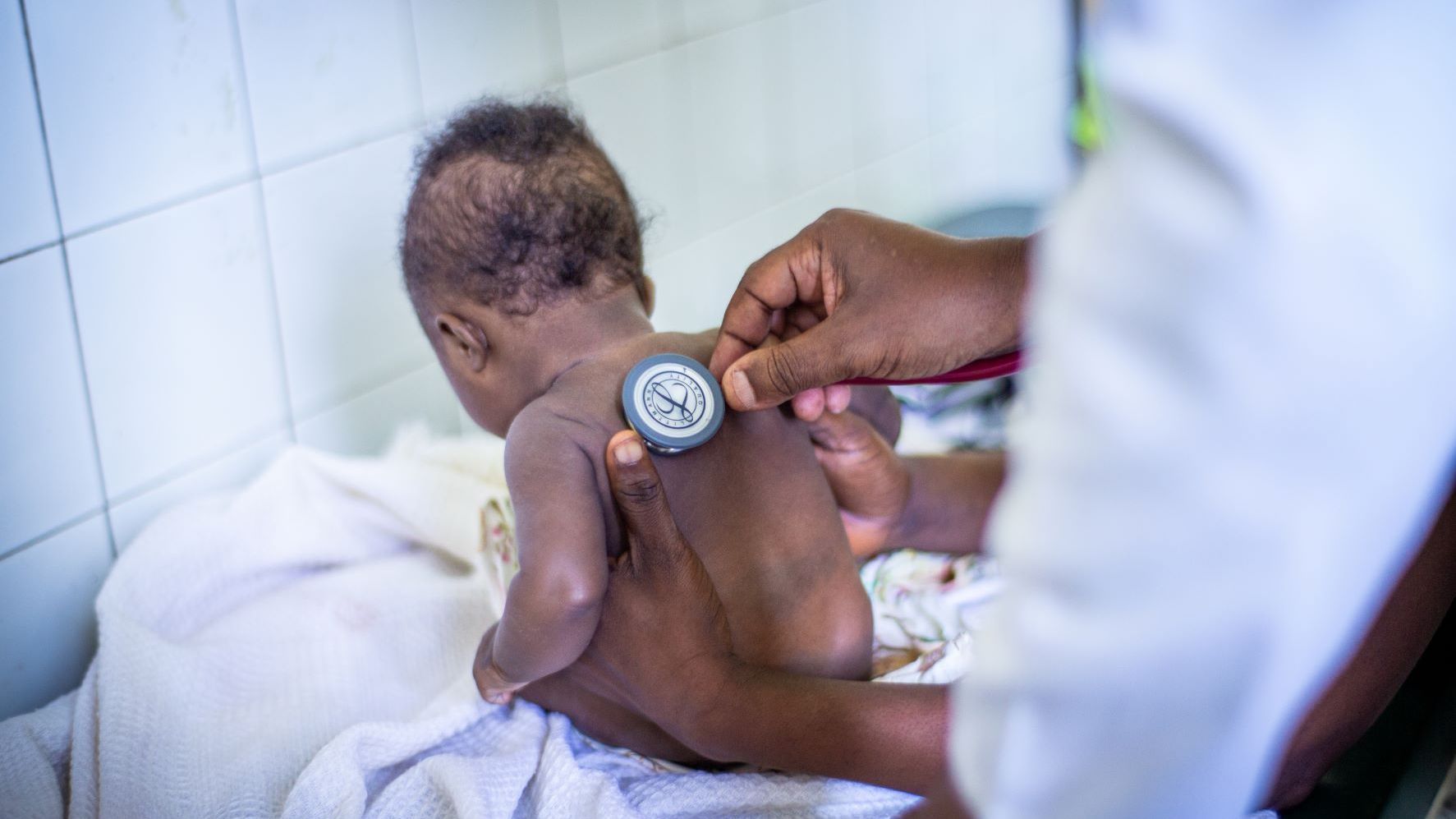
Pediatric Clinical Trials
Children have unique psychosocial, developmental, physical and physiological needs and deserve access to safe and effective medicines. Despite an increase in clinical trials in children due to regulatory requirements such as the Paediatric Investigation Plan introduced in 2006, unlicensed and off-label use of medicines is still common. This unsafe practice can lead to adverse effects, under- or overdosing and potential long-term side effects. Clinical trials in children are therefore essential to develop age-appropriate, validated therapies, paediatric formulations and interventions, and to identify and improve the best available medical care and evidence-based practice. The Clinical Trial Operations Unit has over 15 years of experience in conducting clinical trials in children. With nearly 40% of our trials in children, the fastest growing population in LMICs, we provide expert support in setting up and conducting trials in this population in complex settings, balancing research risks with the need for safe and validated research, and ensuring the highest ethical and clinical standards.


Research Governance and Good Clinical Practice
Promoting evidence-based medicine and conducting clinical research requires customized solutions. We constantly enhance our quality processes and adherence to Good Clinical Practice (GCP). Continuous improvement of our processes is supported by the use of our validated clinical trial management system. Through collaboration with our partners, we offer a wide range of quality services as well as provide Swissethics validated GCP training.



Our Focus
Project and Clinical Study Sites
Strengthening Local Capacities
In low- and middle-income countries, and particularly in rural areas, it may be challenging to ensure sufficient capacity, such as human resources and infrastructure, to facilitate clinical trials up to regulatory standards. Achieving maximum safety and identifying sustainable long-term solutions may require infrastructure development, enhancing local expertise, capacity building and empowering local and existing partners.
The DRC Hub
In order to facilitate our growing number of projects in the Democratic Republic of the Congo (DRC), we established and registered as an NGO in the DRC in July 2015. The office in Kinshasa serves as the local representation of the Clinical Operations Unit and is the basis for a wide range of activities in both clinical research and public health. Swiss TPH also operates a project office in Bukavu, which provides support for capacity building and research projects in the eastern region of the country.
The portfolio of past and current projects in the DRC includes epidemiological research, capacity strengthening activities in public health and malaria, and clinical trials on human African trypanosomiasis (sleeping sickness) and malaria in both paediatric and adult populations.











